




The establishment of advanced cell therapy for the treatment of limbal stem cell deficiency in the Czech Republic
Project ID: TO01000099
Acronym: EYEFORTX-2
Duration: 01/2021 – 04/2024
Funding: This project benefits from € 7 819 grant from Norway Grants and Technology Agency of the Czech Republic within the KAPPA Programme.
The overall EYEFORTX-2 project objective:
Limbal stem cell deficiency (LSCD) affects 3.3 out of 100,000 people in the EU (about 350-500 in
the Czech Republic) and is often leading to a total blindness. The mechanism of the disease, which
The overall project objective:can be triggered by injury, such as chemical or thermal
burn or have genetic etiology is shown in Fig. 1.
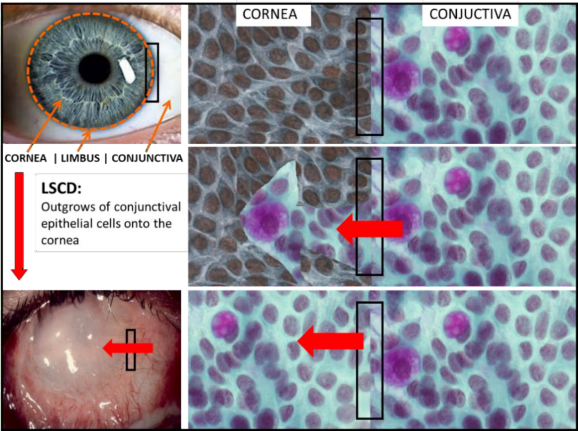
Figure 1. Limbal stem cell deficiency: Conjunctival epithelium extends over corneal surface (red arrows). Such condition originates as a result of progressive depletion or dysfunction of limbal
stem cell population and leads to significant loss of vision or blindness.
Our aim is to restore the vision of patients suffering from both unilateral and bilateral forms of the disease.
Developing cell-based therapy, i.e. the Advanced Therapy Medicinal Products (ATMP) with the
presence of stem cells, requires a considerable funding, experienced top team, facilities such as tissue establishment and clean rooms, a top-quality equipped eye clinic, and rapid transfer of new technology into practice. Any delay in the chain: basic research -> applied research -> product development and approval -> clinical application increases the probability of ending up with
obsolete experimentally developed treatments.
The treatment consists of replacing limbal tissue with the stem cells for the corneal epithelium.
Only the presence of stem cells at the limbus can permanently assure a normal homeostasis, i.e. a
transparent cornea, over which the opaque vascularized conjunctiva does not overgrow. The principle
is schematized in Fig 2.
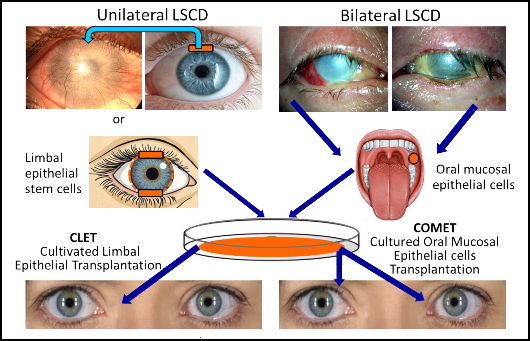
Figure 2. Treatment options for unilateral and bilateral limbal stem cell deficiency (LSCD). In
unilateral LSCD, there is the option for direct transplantation of limbal tissue (blue arrow),
retrieved from the paired healthy eye (risk and disadvantage of causing LSCD), or from a
living-related donor (need for immunosuppression). Another possibility is the Cultivated Limbal
Epithelial Transplantation (CLET), a retrieval of a very small piece of limbus from the healthy
patients' eye and its ex vivo culture to produce a cell sheet which will be used for grafting. In
bilateral LSCD, there are no autologous LECs to retrieve and one option is to retrieve and culture
Oral Mucosal Epithelial Cells (OMECs) ex vivo, and then to transplant them onto corneal surface
(cultivated oral mucosal epithelial transplantation, COMET). Alternatively, other autologous stem
cells,
e.g. hair follicle bulge-derived cells, can be used for such procedure.
The major goal of the project is to speed up the development of ATMP as much as possible and to
start with treatment of unilateral and bilateral. One of the prerequisites for this rapid pace is
the continuity of the results already obtained and the previous cooperation of the partners of the
entire project team. This proposal is a direct continuation of the 'EYEFORTX-2' project, which has received an 'excellent' evaluation and whose results will be partly used in this project. The
majority (65%) of innovation is already available from the latest call from Norwegian grants and follow-up work; some of the results have been further improved and, together with the newly
acquired results, will be transferred to the preclinical (tissue facility – eye and Eye bank Kralovske Vinohrady University Hospital) and clinical practice (transplantation ophthalmological
department of
Kralovske Vinohrady University Hospital).
The establishment of advanced cell therapy for the treatment of limbal stem cell deficiency in the Czech Republic
Papers
Paper 1::
Smeringaiova I, Utheim TP, Jirsova K. Ex vivo expansion and characterization of human corneal endothelium for transplantation: a review. Stem Cell Res Ther. 2021 Oct 30;12(1):554. doi: 10.1186/s13287-021-02611-3. PMID: 34717745; PMCID: PMC8556978. IF(2021): 8.088, Q1, number of citations: 11 (.Result-TO01000099-V4)
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8556978/
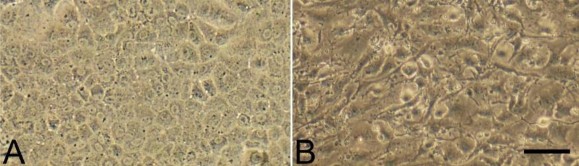
Review that describes the possibilities of the preparation/culture of corneal cells for transplantation purposes. image Corneal endothelial cells cultured for 22 (A) and 25 days (B). A: satisfactory cell morphology, B: cells undergoing endothelial-to-mesenchymal transformation- unsatisfactory morphology.
Paper 2:
Trosan, P., Cabral JV, Smeringaiova I, Studeny P, Jirsova K. Interleukin-13 increases the stemness of limbal epithelial stem cells cultures. PLoS One. 2022 Aug 2;17(8):e0272081. doi: 10.1371/journal.pone.0272081. PMID: 35917378; PMCID: PMC9345474. IF(2022): 3.7, Q2, number of citations: 1 (Result-TO01000099-V8)
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9345474/
The article describes the cultivation of limbal cells with using of interleukin-13.
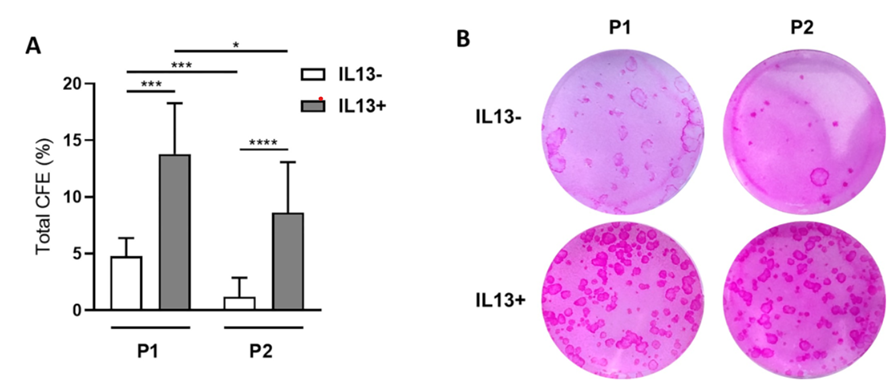
The cultivation of limbal cells has been observed to increase stemness, particularly following the initial passage of cells. Conversely, the cell phenotype undergoes a shift towards a conjunctival phenotype.
Paper 3
El Yamani, N., Rundén-Pran, E., Collins AR, Longhin EM, Elje E, Hoet P, Vinković Vrček I, Doak SH, Fessard V, Dusinska, M. (2022). The miniaturized enzyme-modified comet assay for genotoxicity testing of nanomaterials. FRONTIERS IN TOXICOLOGY, 4. doi:10.3389/ftox.2022.986318. IF(2023): 3.6, Q2, number of citations: 4 (Result-TO01000099-V10)
The development of a miniaturized comet assay (a method for detecting DNA damage) is also being explored for the assessment of the stability and viability of DNA from limbal and oral mucosa cells.
Paper 4
Jeliazkova, N., E. Longhin, N. El Yamani, E. Runden-Pran, Moschini E, Serchi T, Vrček IV, Burgum MJ, Doak SH, Cimpan MR, Rios-Mondragon I, Cimpan E, Battistelli CL, Bossa C, Tsekovska R, Drobne D, Novak S, Repar N, Ammar A, Nymark P, Di Battista V, Sosnowska A, Puzyn T, Kochev N, Iliev L, Jeliazkov V, Reilly K, Lynch I, Bakker M, Delpivo C, Sánchez Jiménez A, Fonseca AS, Manier N, Fernandez-Cruz ML, Rashid S, Willighagen E, D Apostolova M, and M. Dusinska (2024). "A template wizard for the cocreation of machine-readable data-reporting to harmonize the evaluation of (nano)materials.". NATURE PROTOCOLS. doi:10.1038/s41596-024-00993. IF(2023): 13.1, Q1, number of citations: 0; (Result-Others TO01000099-V11)
Link: https://www.nature.com/articles/s41596-024-00993-1
A template for the reporting of data from the comet assay has been developed. This template is suitable for use by the scientific community for the reporting of scientific data, as well as for preclinical studies, including those related to advanced cell therapy. The template has been applied for genotoxicity studies with both limbal and oral mucosal epithelium.
Paper 5
Trousil, J., Cabral JV, Voukali E, Nováčková J, Pop-Georgievski O, Vacík T, Studený P, Studenovska H, Jirsova K. Electrospun poly(l-lactide-co-dl-lactide) nanofibrous scaffold as substrate for ex vivo limbal epithelial cell cultivation. Heliyon. 2024 May 10;10(10):e30970. doi: 10.1016/j.heliyon.2024.e30970. PMID: 38803982; PMCID: PMC11128869, IF3.4, Q1:, number of citations: (Result-TO01000099-V7)
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11128869/
The paper describes the cultivation of limbal cells on the nanofibrous (poly(l-lactide-co-dl-lactide) scaffold.
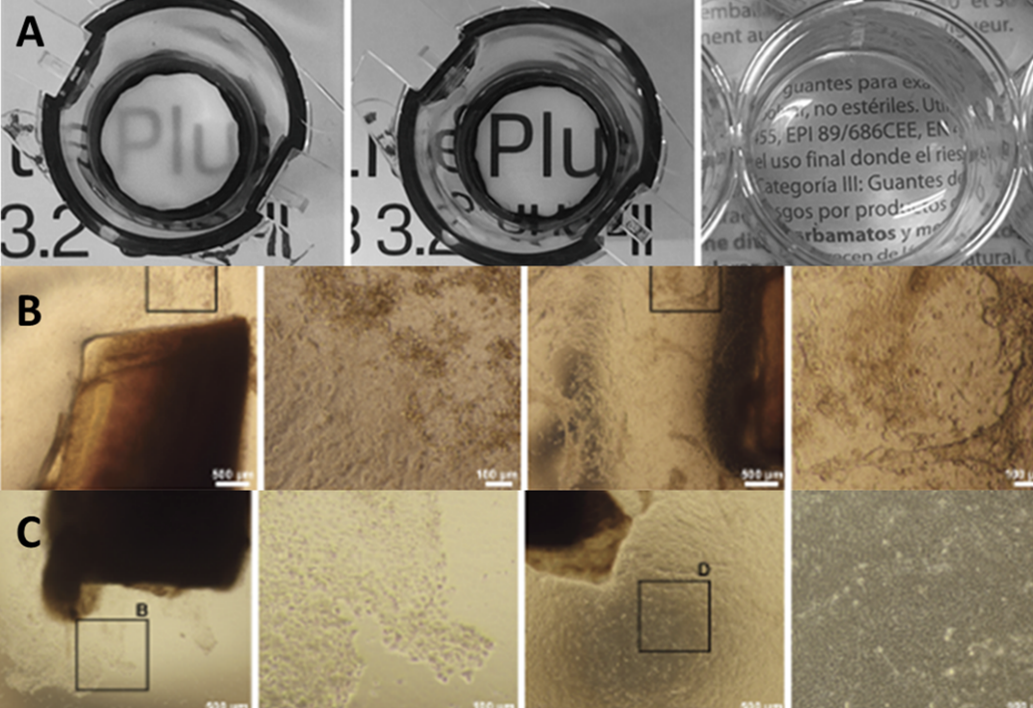
A: transparency of dry and wet poly(l-lactide-co-dl-lactide), and fibrin, B: cultivation of limbal cells on poly(l-lactide-co-dl-lactide), cultivation of limbal cells on fibrin glue
Those involved in TACR the project are highlighted in bold.
The total IF of the articles published so far in the project reaches 31.9.
Submitted manuscripts:
• DNA damage in oral mucosal epithelial cells cultured in complex and xenobiotic-free media: A comparison study. V. Cabral, S. Vodenkova, K. Tomasova, N. El Yamani, N., E. R. Pran, M. Nekutova, A. Safanda, M. Dusinska, L. Vodickova, K. Jirsova, submitted to Mutagenesis, Result-TO01000099-V2
• Effect of Cryoprotectants on Long-Term Storage of Oral Mucosal Epithelial Cells: Implications for Stem Cell Preservation and Proliferation Status. Joao Victor Cabral, Natalia Smorodinova, Eleni Voukali, Pavel Studeny, Vojtěch Kolín, Katerina Jirsova, submitted to Acta Biologica, Result-TO01000099-V3
• Cultivation and characterization of oral mucosal epithelial cells on fibrin gel in a xeno-free medium for the treatment of Limbal Stem, J. V. Cabral, E. Voukali, N. Smorodinova, L.Balogh, V. Kolín, M. Netuková, P. Studeny, K. Jirsova submitted to Experimental Eye Research, Result-O01000099-V5
• Eleni Voukali, Joao Victor Cabral, Natalia Smorodinova, Tomáš Vacík, V. Kolín, Katerina Jirsova: Comparison of angiogenic and inflammatory factor gene expression between limbal and oral mucosal epithelial cells in vitro, submitted to International Journal of Molecular Sciences Manuscript ID: ijms-3083608, type of manuscript: Article Results-Others-TO01000099-V12
OTHER RESULTS
Methods:
Result-TO01000099-V1: LECs: set of protocols
Result-TO01000099-V6: OMECs: set of protocols
EYEFORTX-2 Project News
2023:
09.10.2023: João Victor de Sousa Cabral, MD. Successful Defence of Doctoral Dissertation, Study programme: Cell Biology and Pathology
Title: Regenerative Medicine in Ocular Surface Reconstruction: Advancing Cell-Based Therapies for Limbal Stem Cell Deficiency

Photos from doctoral graduation May 2024 Karolinum, Charles University.
2024:
19.04.2024 - 20.04.2024, Rostoc
6. Baltic Sea Eye Conference
Two members of the project team, prof. K. Jirsová, PhD., and Dr. Eleni Voukali, PhD., attended this scientific ophthalmology conference at the invitation of the main organizer, prof. Thomas A. Fuchsluger.
The following lectures were given:
• The cultivation of oral mucosal epithelial cells for consequent clinical use, authors: Katerina Jirsova, Victor Cabral, Eleni Voukali, Natalia Smorodinova
• Comparison of angiogeneic factors gene expression between oral mucosal epithelial cells and limbal epithelial cells, autors: Eleni Voukali, Victor Cabral, Natalia Smorodinova, Tomas Vacik a Katerina Jirsova
The remarkable achievement of Dr. Eleni Voukali, PhD. and the whole team was winning the main prize in the category for young investigators section
Testimonials
Vestibulum sem purus, interdum sit amet varius id, porta sit amet sem. Mauris nec dui ut sapien finibus bibendum eget sit amet nulla. Proin condimentum urna quis tincidunt euismod. Vivamus eget sem at lorem varius pretium. Sed nisl lacus, sollicitudin sed elit et, convallis convallis arcu.
Nulla ut consequat felis, et euismod enim. Etiam at tellus eget dui tristique eleifend pretium eu tellus. Integer eu facilisis velit. Proin in mollis metus, id vestibulum enim.
Contact
Project Partners:
Laboratory of the Biology and Pathology of the Eye, Institute of Biology and Medical Genetics, First Faculty of Medicine, Charles University and General University Hospital in Prague,
Prof. Katerina Jirsova, PhD.
Department of Ophthalmology, University Hospital Kralovske Vinohrady, Prague,
Assoc. Prof. Pavel Studený, MD. Ph.D.
Institute of Macromolecular Chemistry, Czech Academy of Sciences (IMC), Prague,
Ing. Hana Studenovská Ph.D.
The Norwegian Institute of Air Research,
Prof. Dr. Mária Dušinská, Ph.D. Oslo University, Catherine Jackson Ph.D.
Our team consists of: scientists (cell and molecular biologists from CUNI (K. Jirsova, V. Cabral) and OU; scientists responsible for vitrification and long term storage of cells – J. Bednar, CUNI; scientists responsible for genome stability testing – NILU (M. Dusinska, N. El Yamani, H. Fjeldstad); scientists responsible for new material for cell cultivation and their transfer into a diseased eye (H. Studenovska). pre-clinicians (tissue and cell bankers, K. Jirsova, M. Netukova) experienced clinicians M. Netukova and P. Studeny from KVUH. The pre- clinicians and clinicians, together with the scientists, will be responsible for developing a procedure for applying cultured cells to the eye surface, selecting patients for treatment, ATMP transplantation, screening, and follow-up. They will also be responsible for the analysis and subsequent publication of the study results. The team also includes other experienced scientists, PhD. students, lab technicians and administrators. Cooperation with CJ. Jackson, Oslo University will also lead to the transfer of experience with cultivation methods, preparation and storage procedures that would have to undergo long-term development without such cooperation.
Contacts: Principal Investigator: Katerina Jirsova, PhD., katerina.jirsova@lf1.cuni.cz, tel: 0022496 8006
Project Administrator: Kristyna Matejkova, DiS. kristyna.matejkova@lf1.cuni.cz, 0022496 4264
Links: KAPPA funding programme for applied research, experimental development and innovation: https://www.tacr.cz/en/kappa-programme
Project Promoter: www.cuni.cz
Project Information: https://starfos.tacr.cz/en/project/TO01000099#project-main
Albertov 4, 128 00 Prague, Czech Republic

